Introduction
Chronic Hepatitis B virus (HBV) infection remains a global health challenge, affecting over 250 million individuals worldwide. Among these, a significant portion are inactive HBV carriers — individuals who are HBsAg-positive, HBeAg-negative, with low or undetectable HBV DNA levels and normal liver enzymes. Although they exhibit minimal viral activity, they still face long-term risks of liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC).
This article highlights a case of MSC therapy in an inactive HBV carrier, exploring how mesenchymal stem cells (MSCs) can aid in maintaining liver health, modulating the immune system, and improving quality of life. We also review current scientific evidence on the safety and efficacy of MSCs in this context.
Case Summary: Mr. T – A 59-Year-Old Inactive Hepatitis B Carrier
Mr. T, a 59-year-old male, presented as an inactive HBV carrier. His recent laboratory findings included:
- HBsAg: Reactive (Positive)
- HBeAg: Non-reactive (Negative)
- Anti-HBe: Reactive (Positive)
- HBV DNA Viral Load: 755 IU/mL (Low)
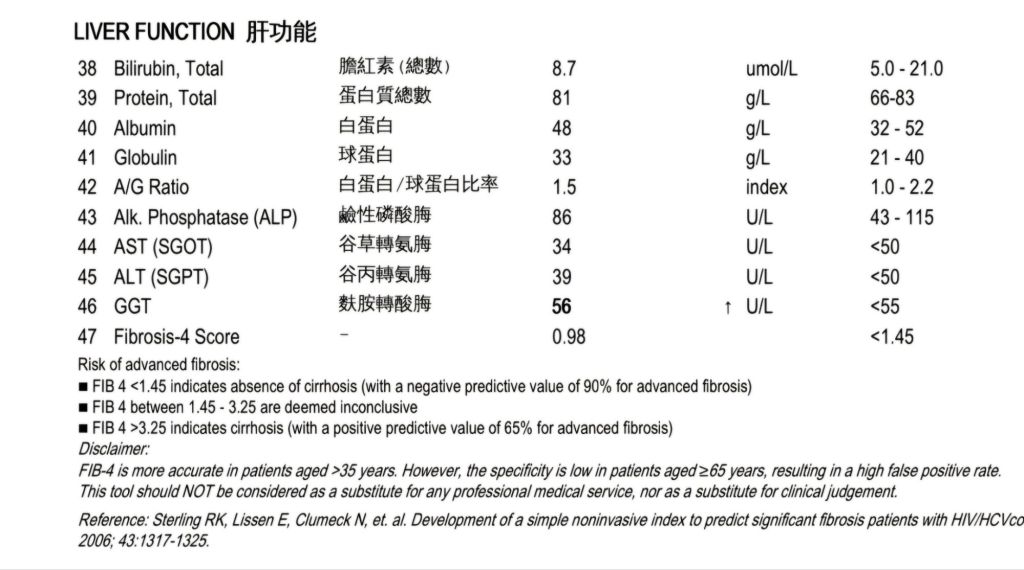
- Liver Function: Normal


Mr. T did not exhibit symptoms or signs of active liver disease. However, due to concerns about long-term liver health and a desire to improve his energy levels and overall wellness, he elected to undergo intravenous mesenchymal stem cell therapy.

Why Consider MSC Therapy in Inactive Hepatitis B Carriers?
While antiviral therapy is not typically indicated for inactive carriers, MSC therapy offers a regenerative and immunomodulatory approach to support liver health and prevent disease progression. MSCs are not antiviral agents but have demonstrated anti-inflammatory, anti-fibrotic, and liver-regenerating properties, which may benefit HBV carriers over time.
Goals of MSC Therapy in This Case:
- Prevent progression of liver fibrosis or cirrhosis.
- Enhance liver regeneration and cellular repair.
- Modulate immune responses to maintain low viral activity.
- Support general vitality, reduce fatigue, and improve well-being.
Mr. T received 100 million (1 × 10⁸) MSCs intravenously in a controlled clinical setting with regular HBV DNA monitoring. No antiviral therapy was initiated due to his low viral load and inactive disease status.
Scientific Basis: How MSCs Benefit HBV Carriers
1. Immune Modulation
MSCs regulate the immune system by:
- Increasing regulatory T cells (Tregs).
- Reducing pro-inflammatory cytokines (IL-6, TNF-α).
- Enhancing anti-inflammatory cytokines (IL-10, TGF-β).
This immune balance helps prevent immune-mediated liver injury, a key driver of fibrosis in HBV infection.
2. Anti-Fibrotic and Regenerative Effects
MSCs secrete growth factors and extracellular vesicles that:
- Inhibit hepatic stellate cell activation (key fibrosis mediators).
- Stimulate liver progenitor cells and hepatocyte regeneration.
- Enhance angiogenesis and tissue oxygenation.
3. Safety in HBV Carriers
Concerns about HBV reactivation due to MSC immune modulation are valid. However, clinical studies report no HBV reactivation events in HBsAg-positive patients treated with MSCs, especially when antiviral prophylaxis or monitoring is in place.
In vitro studies even suggest MSCs might suppress HBV replication in immune co-culture systems.
Relevant Clinical Studies
- Wang et al., 2013 – Stem Cells and Development
- Study: Umbilical MSCs in HBV-related cirrhosis.
- Results: Improved liver function (MELD score, albumin), no HBV reactivation.
- Conclusion: MSC therapy is safe and beneficial in chronic HBV patients.
- Lin et al., 2017 – Journal of Translational Medicine
- Study: MSCs in HBV-associated acute-on-chronic liver failure.
- Results: Reduced inflammatory markers, improved survival.
- Conclusion: MSCs modulate immune response favorably in HBV context.
- Peng et al., 2011 – Journal of Gastroenterology and Hepatology
- Study: Autologous MSCs in HBV cirrhosis.
- Results: Safe with positive liver outcome trends.
- Note: Highlights long-term potential of MSC therapy.
- Xie et al., 2016 – Virology Journal
- Study: MSCs co-cultured with lymphocytes in HBV model (in vitro).
- Results: Suppressed HBV DNA and cccDNA levels.
- Conclusion: Potential MSC-mediated antiviral effect.
- Zhou et al., 2014 – PLOS One
- Study: HBV infectivity in MSCs from different sources.
- Results: Adipose-derived MSCs resist HBV infection.
- Safety Insight: Supports use of certain MSC types in carriers.
Conclusion and Outlook
Mr. T’s treatment represents a proactive approach to liver health in chronic HBV carriers. With no adverse effects and stable viral load, MSC therapy provided him with a sense of renewed energy and peace of mind regarding his liver’s future.
MSC therapy does not replace antiviral medication, but it may offer adjunctive benefits in inactive HBV carriers aiming for long-term liver wellness and immune balance.
Future research will help define optimal patient selection, long-term benefits, and cost-effectiveness of MSC use in HBV carriers. For now, early evidence and patient experiences like Mr. T’s are encouraging.
Disclaimer
MSC therapy is not yet standard care for Hepatitis B and should be considered under specialist guidance with proper monitoring. Consult your healthcare provider before pursuing advanced cellular therapies.